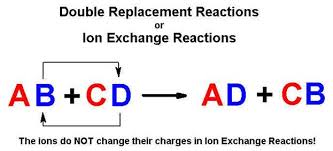
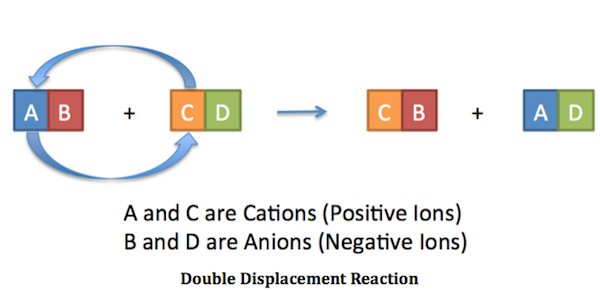
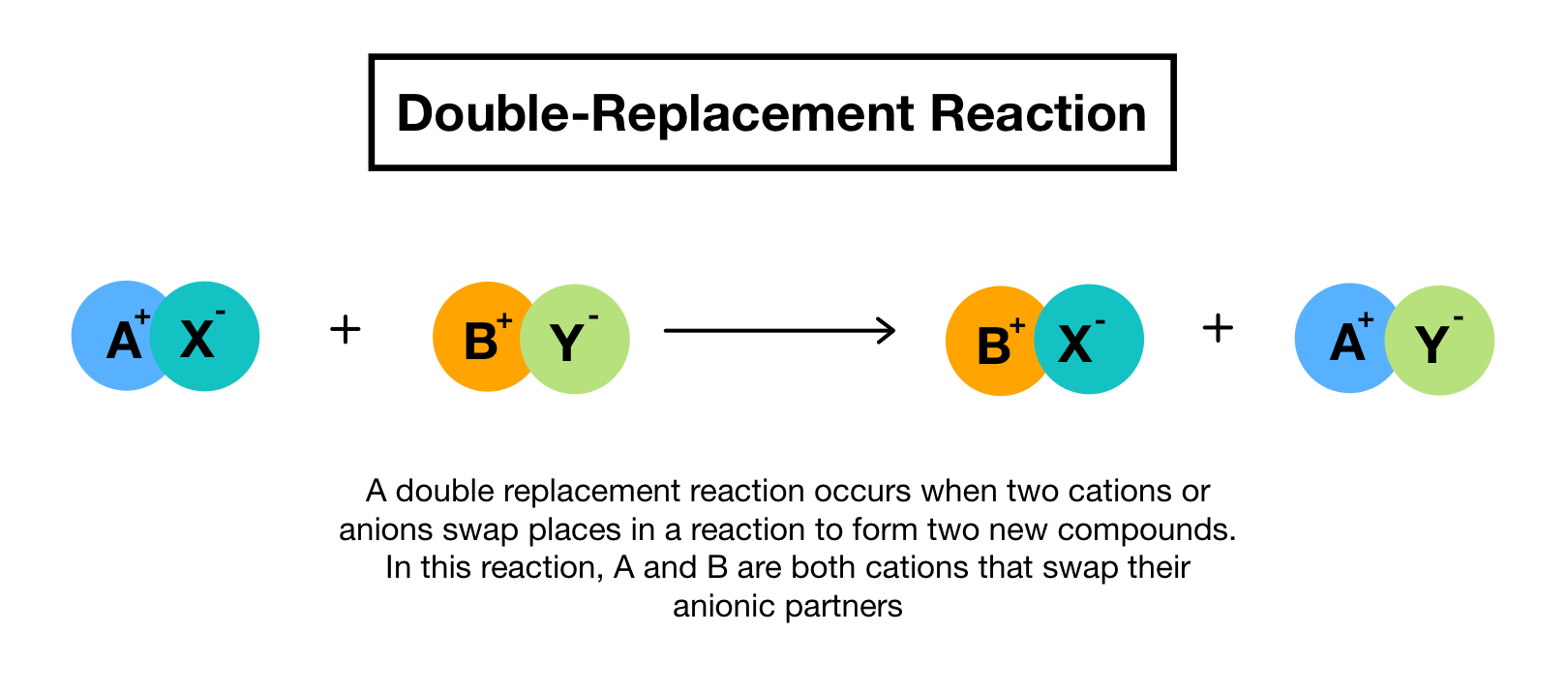
Double replacement sometimes referred to as double displacement reactions are when parts of ionic compounds are switched to form two new ionic compounds. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products.
What Is A Double Replacement Reaction In Chemistry Socratic
This chemistry video tutorial explains how to identify the products of a double replacement reaction from a sentence or word problem.

. Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. Usually in these reactions when combining aqueous solutions a solid product is also formed. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds.
Two elements reacting to form a single compound. 4ja1 4jb1 4jb1t 4jb1tc diesel engine EXPERIMENT 5 Double Replacement. Double replacement reactions are also called metathesis or double displacement reactions.
Two examples are also sho. Reaction Type and Balancing Worksheet - Key. Reactants Products Many of these reactions occur in an aqueous environment ie in a solution where ions and compounds.
This product is referred to as a precipitate and the reaction is called a precipitation reaction. In this printable students also use an equation to answer questions about elements and chemical reactions. Solubility rules are used to predict whether some double-replacement reactions will occur.
A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative. Chemistry double replacement reactions lab answers june 23rd 2018 - read and download chemistry double replacement reactions lab answers free ebooks in pdf format mergerstat control premium study 2013 isuzu 4j 1 2. Chemistry Chemical Reactions Double Replacement Reactions 1 Answer Denise Granger Jan 28 2017 The clue lies in the name itself.
A double-replacement reaction occurs when parts of two ionic compounds are exchanged making two new compounds. Many double displacement reactions occur between ionic compounds that are dissolved in water. Two compounds react to form one new compound.
A double replacement reaction will occur if a formation of a precipitate gas or water takes place. Double displacement reactions occur when two ionic compounds react or when an ionic compound reacts with an acidu000bu000b It only occurs in liquids. D What are the spectator ions in.
A double-replacement reaction exchanges the cations or the anions of two ionic compounds. One compound reacts to form two separate elements. The reaction occurs most often between ionic compounds although technically the bonds formed between the chemical species may be either ionic or covalent in nature.
It covers three types. Question 1 120 seconds Q. The truefalse questions in this worksheet will help students review the process of a double-replacement reaction.
Chemistry double replacement reaction worksheet answers that we will certainly offer. It is not vis--vis the costs. The way I think of it since were dealing with ionic compounds is that when I write out a reaction I.
Predict products for single replacement reactions How to predict products for double replacement precipitate reactions Types of Chemical Reactions Predicting Products Worksheet Answer Key Learning to mathematically analyze circuits requires much study and practice. For example take a look at this reaction. NH42SO4aq CaBr2aq a Predict the products and their phases b Write a complete ionic equation for the reaction.
A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds. What Is a Double-Replacement Reaction. Science Chemistry QA Library For the following double-replacement reaction.
What is double decomposition give an example. In this lab double replacement reactions between compounds were done in order to determine the equation and description of a new substance. How are double replacement reactions performed in the lab.
Its practically what you craving currently. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. What is a double replacement.
Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. C Write the net ionic equation for the reaction. Answer choices Two compounds react to form two new compounds.
In double replacement reactions the positive ions exchange negative ion partners. Question 2 120 seconds Q. Double Replacement Reactions One of the main purposes of chemistry is to transform one set of chemicals the reactants into another set of chemicals the products via a chemical reaction.
Describes the basics of double replacement reactions how to identify them predict the products and balance the chemical equation. An example is CuCl2aq 2 AgNO3aq Cu NO32aq 2 AgCl s. Select two compounds above and this calculator will predict whether or not the reaction will occur in water.
The overall pattern of a double replacement reaction looks like this. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. This is simply based on the solubility chart of inorganic compounds.
This chemistry double replacement reaction worksheet answers as one of the most effective sellers Page 112. A double displacement reaction is also called a double replacement reaction salt metathesis reaction or double decomposition.
Double Replacement Reactions Chemistry Socratic

Double Replacement Double Displacement Reaction

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
Double Replacement Reactions Definition Examples Expii

Double Replacement Reaction Definition And Examples
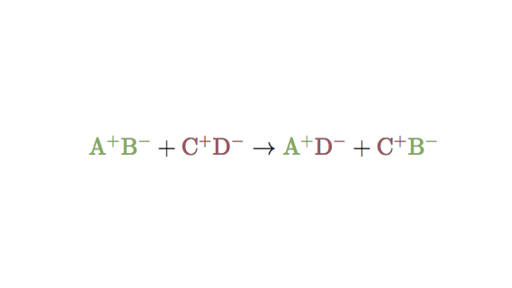
Double Replacement Reactions Double Displacement Article Khan Academy

Single Replacement Reaction Definition And Examples

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
0 comments
Post a Comment